Spinal Testing
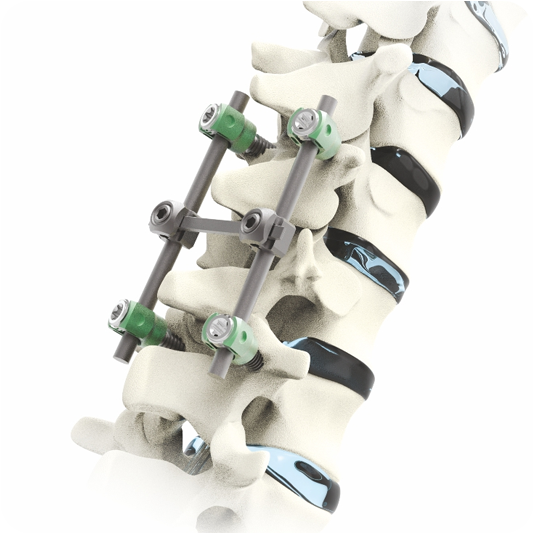
Spinal Implants Testing
Our spinal implants testing capabilities extend to fusion and non-fusion spinal devices, as well as predicate components, for feasibility testing and regulatory submission requirements.
Spinal implants can experience a variety of forces within the human body, so ensuring their durability and lifespan is important for the well-being of the user. Our trusted medical device testing allows your company to mitigate risk knowing that we are working diligently to help ensure your success and bring a safe product to market.
Common mechanical testing for spinal implants includes static and fatigue tests in different loading modes, such as axial, torsional, shear, flexion/extension, bending, lateral bending, and axial rotation.
Spinal implants are categorized into two groups: fusion devices and non-fusion devices. Spinal cages and stabilization systems are typically used to treat fractures, disc compression, and deformities, whereas non fusion devices are used to maintain spine mobility while relieving pressure.
Standard Tests & Guides
- ASTM F1717: Standard Test Methods for Spinal Implant Constructs in a Vertebrectomy Model
- ASTM F1798: Standard Guide for Evaluating the Static and Fatigue Properties of Interconnection Mechanisms and Subassemblies Used in Spinal Arthrodesis Implants
- ASTM F2077: Test Methods for Intervertebral Body Fusion Devices
- ASTM F2193: Standard Specifications and Test Methods for Components Used in the Surgical Fixation of the Spinal Skeletal System
- ASTM F2267: Standard Test Method for Measuring Load Induced Subsidence of an Intervertebral Body Fusion Device under Static Axial Compression
- ASTM F2346: Standard Test Methods for Static and Dynamic Characterization of Spinal Artificial Discs
- ASTM F2423: Standard Guide for Functional, Kinematic, and Wear Assessment of Total Disc Prostheses
- ASTM F2624: Standard Test Method for Static, Dynamic, and Wear Assessment of Extra-Discal Spinal Motion Preserving Implants
- ASTM F2694: Standard Practice for Functional and Wear Evaluation of Motion-Preserving Lumbar Total
